不良事件 AE / 严重不良事件 SAE
一、不良事件
 不良事件: 指受试者接受试验用药品后出现的所有不良医学事件,可以表现为症状体征、疾病或者实验室检查异常,但不一定与试验用药品有因果关系。1)
不良事件: 指受试者接受试验用药品后出现的所有不良医学事件,可以表现为症状体征、疾病或者实验室检查异常,但不一定与试验用药品有因果关系。1)
Adverse Event (AE): Any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment. An adverse event (AE) can therefore be any unfavourable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medicinal (investigational) product, whether or not related to the medicinal (investigational) product.2)
临床试验中,不良事件一般从签署知情同意书开始记录。
一个完整的不良事件记录,通常需要包含的信息:3)
- 名称(AE Term)
- 开始时间(Date of Onset)
- 结束时间(Date of Outcome)
- 程度(Intensity)
- 药物相关性(Relationship)
- 采取的措施(Action)
- 是否用药(Concomitant Medication)
- 转归(Outcome)
不良事件相关性
1. WHO 乌普萨拉中心国际药品ADR监测合作中心
| 序号 | 评定 | 说明 |
|---|---|---|
| 1 | Certain | a clinical event, including laboratory test abnormality, occurring in a plausible time relationship to drug administration, and which cannot be explained by concurrent disease or other drugs or chemicals. The response to withdrawal of the drug (dechallenge) should be clinically plausible. The event must be definitive pharmacologically or phenomenologically, using a satisfactory rechallenge procedure if necessary. |
| 2 | Probable/Likely | a clinical event, including laboratory test abnormality, with a reasonable time sequence to administration of the drug, unlikely to be attributed to concurrent disease or other drugs or chemicals, and which follows a clinically reasonable response on withdrawal (dechallenge). Rechallenge information is not required to fulfil this definition. |
| 3 | Possible | a clinical event, including laboratory test abnormality, with a reasonable time sequence to administrations of the drug, but which could also be explained by concurrent disease or other drugs or chemicals. Information on drug withdrawal may be lacking or unclear. |
| 4 | Unlikely | a clinical event, including laboratory test abnormality, with a temporal relationship to drug administration which makes a causal relationship improbable, and in which other drugs, chemicals or underlying disease provide plausible explanations. |
| 5 | Conditional/Unclassified | a clinical event, including laboratory test abnormality, reported as an adverse reaction, about which more data is essential for a proper assessment, or the additional data is under examination. |
| 6 | Unassessable/Unclassifiable | a report suggesting an adverse reaction which cannot be judged because information is insufficient or contradictory, and which cannot be supplemented or verified. |
二、严重不良事件
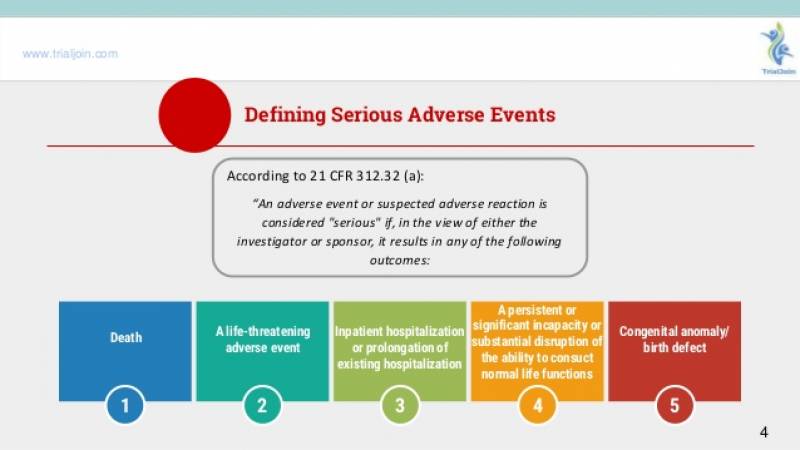 严重不良事件: 指受试者接受试验用药品后出现死亡、危及生命、永久或者严重的残疾或者功能丧失、受试者需要住院治疗或者延长住院时间,以及先天性异常或者出生缺陷等不良医学事件。4)
严重不良事件: 指受试者接受试验用药品后出现死亡、危及生命、永久或者严重的残疾或者功能丧失、受试者需要住院治疗或者延长住院时间,以及先天性异常或者出生缺陷等不良医学事件。4)
Serious Adverse Event (SAE) or Serious Adverse Drug Reaction (Serious ADR):Any untoward medical occurrence that at any dose:5)
- results in death
- is life-threatening
- requires inpatient hospitalization or prolongation of existing hospitalization
- results in persistent or significant disability/incapacity,
- or is a congenital anomaly/birth defect
(一) 报告对象及时限:
- 另外,卫生部2011年的时候颁布了一份《药品不良反应报告和监测管理办法》,主要是针对药品上市后监管的。其中有如下规定:
- 第二十一条 药品生产、经营企业和医疗机构发现或者获知新的、严重的药品不良反应应当在15日内报告,其中死亡病例须立即报告;其他药品不良反应应当在30日内报告。有随访信息的,应当及时报告。
- 药品生产企业应当对获知的死亡病例进行调查,详细了解死亡病例的基本信息、药品使用情况、不良反应发生及诊治情况等,并在15日内完成调查报告,报药品生产企业所在地的省级药品不良反应监测机构。
- 第三十三条 进口药品和国产药品在境外发生的严重药品不良反应(包括自发报告系统收集的、上市后临床研究发现的、文献报道的),药品生产企业应当填写《境外发生的药品不良反应/事件报告表》(见附表3),自获知之日起30日内报送国家药品不良反应监测中心。国家药品不良反应监测中心要求提供原始报表及相关信息的,药品生产企业应当在5日内提交。
- 第三十五条 进口药品和国产药品在境外因药品不良反应被暂停销售、使用或者撤市的,药品生产企业应当在获知后24小时内书面报国家食品药品监督管理局和国家药品不良反应监测中心。
- 第三十七条 设立新药监测期的国产药品,应当自取得批准证明文件之日起每满1年提交一次定期安全性更新报告,直至首次再注册,之后每5年报告一次;其他国产药品,每5年报告一次。首次进口的药品,自取得进口药品批准证明文件之日起每满一年提交一次定期安全性更新报告,直至首次再注册,之后每5年报告一次。定期安全性更新报告的汇总时间以取得药品批准证明文件的日期为起点计,上报日期应当在汇总数据截止日期后60日内。
(二) 报告表格:
各个药厂有各自的严重不良事件报告表。国内官方的严重不良事件报告表有两个版本:
- 2000年药监局发布的《药品临床研究的若干规定》里的附表2。
- 总局药品评价中心,即国家药品不良反应监测中心 网站上的《上市前临床试验严重不良事件报告表》。
其中,第二个表格是被广泛使用的。
(三) 报告方式:
1. 严重不良事件报告给国家药品监督管理局药品注册司:
- 首选通过传真报告:
- 传真号码: 010-8836 3228
- 器械SAE传真号码: 010-6858 6295
- 如遇无法传真,须以EMS方式邮寄纸质报告:
- 地址: 北京市西城区宣武门西大街26号院2号楼,国家食品药品监督管理局注册司研究监督处
- 邮编: 100053
- 保留好传真失败记录,及EMS快递单底单。
2. 严重不良事件报告给国家药品监督管理局安全监管司:
???
3. 省、自治区、直辖市药品监督管理部门:
(待完成)
4. 疫苗临床试验严重不良事件报告:
2014年药监局发布了《疫苗临床试验严重不良事件报告管理规定(试行)》。其中规定了,可疑且非预期严重不良反应(SUSAR)等个案报告以及定期安全性报告由申办者按《规定》向总局药品审评中心报送。并以纸质 和 电子方式报告给总局药品审评中心资料组:
- 纸质报告以快递的形式寄送,用于存档:
- 收件人:国家食品药品监督管理总局药品审评中心资料组
- 地址:北京市海淀区复兴路甲1号
- 邮编:100038
- 纸质报告须附临床试验批件复印件,并加盖公章。
- 电子报告可选择传真或电子邮箱:
- 传真号码:010-68584220
- 电子邮箱:susar@cde.org.cn。
三、其他相关概念
治疗期间出现的不良事件(Treatment Emergent Adverse Event,TEAE):在治疗时出现的,而在治疗前没有的或比状况更坏的不良事件。多用于临床试验统计报告中,定义来自ICH指南E9《临床试验统计原则》中。9)
药物不良反应(Adverse Drug Reaction,ADR):在一个新的药品或药品的新用途在批准之前的临床实践,尤其是治疗剂量尚未确定前,ADR是指与药物任何剂量有关的所有有害的和非预想的反应都应被考虑为药品不良反应。该术语用于药品是指在药品与不良反应之间的因果关系至少有一个合理的可能性,即不能排除这种关系。对已上市药品,ADR指人对用于预防、诊断或治疗疾病或改善生理功能的药物在常用剂量出现的有害和非预想的反应。10)
药品不良反应: 是指合格药品在正常用法用量下出现的与用药目的无关的有害反应。11)
重要不良事件(Significant Adverse Event):指的是除严重不良事件外,发生的任何导致采用针对性医疗措施(如停药、降低剂量和对症治疗)的不良事件和血液学或其他实验室检查明显异常。12)
四、扩展阅读
- 《药品不良反应知识100问》. 国家药品不良反应监测中心. 201113)
- 药物临床试验不良反应/不良事件关联性判定方法研究探讨. 李博,高蕊,李睿等. 中国新药杂志, 2014年, 第23卷第12期
五、相关讨论
1. 提问:化验单中的异常值什么情况下判定为有临床意义?什么情况下的异常值需要报AE?
回答:
2. 提问:
回答:
SAEA的上报流程要更新下了吧,根据2020新版GCP,大部分SAE已经不需要24小时上报了